
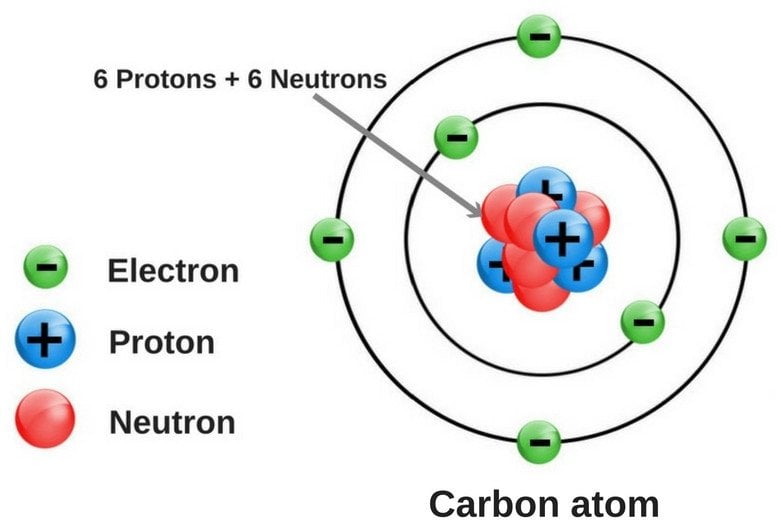
If we look at an X-ray diffraction-based structure of diamond, we find that each carbon atom is surrounded by four other carbon atoms situated at equal distances and equal angles from each other.

If the diamond were not cut with so many facets it would allow most light to pass through it. A polished diamond is sparkly because some light is reflected from the surface and some light passes through it - making it transparent.

CARBON ELECTRONIC STRUCTURE FREE
Since it doesn’t conduct electricity the electrons must not be free to move around within a diamond. Since diamond is so stable (chemically inert), it must have very strong bonds that take a lot of energy to break. Let us step back and look at the properties of diamond and see if we can make sense of them. Any useful model of diamond’s structure must explain how these properties arise from atomic interactions. This suggests that the molecular level structure of diamond is quite different from that found in metals (which as we will see are malleable and conduct electricity). Diamonds are extremely hard (the hardest naturally occurring substance) and do not conduct electricity at all (to conduct electricity, electrons must be able to flow through the material). There is no such thing as molten diamond. If it is heated in an atmosphere of oxygen it will react to produce carbon dioxide if oxygen is absent it will transform into graphite - another allotrope of carbon (see below). In addition, when diamond is melted it decomposes. Diamond has the highest melting point of any known substance, so high that these measurements are actually done under high pressure and then calculated to estimate what the value would be at atmospheric pressure. That is: it is very hard to make diamonds do anything at all except sit there and sparkle – they don’t dissolve in water and they melt only at very high temperatures (mp 3330☌ ). Diamonds are so valued because they are rare, sparkly, hard, and almost completely inert. Diamonds have also been found in asteroids, which originate from outside of the earth. Such conditions can be found about 100 miles under the Earth’s crust, the region known as the lithosphere. Diamond is the name given to one of the naturally occurring forms (allotropes) of pure C the other allotropes of carbon are graphite, graphene, and various fullerenes (FIGURE BELOW) – which we will return to later.ĭiamonds form from carbon rich materials subjected to very high pressure (45,000-60,000 atmospheres) but relatively low temperatures (900-1300 ✬). While this is the most common model, we will see that it is not the only possible one we will introduce other models as they are needed. Each bond involves two electrons (one from each of the bonded atoms). The bonding between C atoms (and to other types of atoms) is typically described using the covalent bonding model. Carbon (C) belongs to the family of elements known as non-metals. For starters, let us take a look at carbon. In order to give you an idea of some of the different types of bonds that form between elements we are going to consider several representative elements from different areas of the periodic table.

Chemistry, life, the universe and everything Chapter 3.3: Carbon - an amazingly allotropic element


 0 kommentar(er)
0 kommentar(er)
